Describe Electronegativity in Your Own Words
What do chemists mean by the term electronegativity. In this lab you will work to answer a number of questions.

What Is Electronegativity Trends Chart Periodic Table Chemtalk
So electro negativity is the tendency oven Adam to attract those electrons and electron affinity is that energy that is released.

. Be sure your answer has a unit symbol if. Electronegativity is the tendency of an atomelement to attract a shared pair of electrons towards itself. BIU A А IX EX X SE 12pt Paragraph Next Previous.
Add To Playlist Add to Existing Playlist. 5 points 7. 5 points The electronegativity of Atom A is at less while the electronegativity of Atom B is in the middle of less and more.
What are the four most electronegative elements and what does electronegativity mean describe in your own words. The tendency of an atom in a molecule to attract the shared pair of electrons towards itself is known as electronegativity. What are the partial charges at these electronegativities.
What are the 0122. Electronegativity is the property of an atom which increases with its tendency to attract the electrons of a bond. Transcribed image text.
Electronegativity refers to an atoms ability to attract the electrons present in a chemical bond or an atoms ability to attract electrons when that atom is part of a specific compound. In your own words explain what the Partial Charges and Bond Character button display. In most cases the electrons found within a chemical bond have a greater attraction to one atom than to the other atom which creates a polar covalent bond.
Electronegativity is affected by the atomic number and the distance between the valence electrons and its nucleus. So its referring to the energy when those electrons are added to those atoms. The bond dipole is pointing in the right direction the direction of the more negative atom atom B.
In your own words what is meant by the term electronegativity. Lastly explain why the noble gases are not assigned electronegativity values. It basically indicates the net result of the tendencies of atoms in different elements to attract the bond-forming electron pairs.
At what electronegativities for both Atom A and B is the bond character most likely to be covalent. Use complete sentences to answer. Use complete sentences to answer.
Up to 24 cash back Electronegativity is a measure of a single atoms ability to attract the electrons shared in that bond. The repulsion between electron pairs increases with an increase in electronegativity of the central atom and hence the. The polarity of a bond is affected by the electronegativity values of the two atoms involved in that bond.
Describe electronegativity trends in the periodic table. Also were asked to describe how electro negativity ease uh change according to the elements position on the periodic table. If two bonded atoms have the same electronegativity values as each other they share electrons equally in a covalent bond.
The higher the electronegativity is the more it attracts the electrons towards it. Create a New Plyalist. How does electronegativity increasedecrease within a group and period in the periodic table.
It is a dimensionless property because it is only a tendency. Electronegativity is a chemical property that measures the tendency of an atom to attract electrons towards itself. 2A molecule can possess polar bonds and still be nonpolar.
We review their content and use your feedback to keep the quality high. Use complete sentences to answer. Now as Cl is more electronegative than H it will pull the electron of H towards itself more.
Sulfide required to make 3kg of copper. 27 6 points In your own words describe the concept of electronegativity. 1The electronegativity of an atom determines how strongly it attracts electrons to itself.
From what youve already learned about the protons and electrons in an atom what would cause an atom to have a high electronegativity value. Find an answer to your question Briefly explain in your own words why the bond angle increases as the number of electron groups decreases. Suppose A bond is formed as a result of chemical reaction between H and Cl and electrons are shared.
Usually the electrons in a chemical bond are more attracted to one atom the more electronegative one than to the other.

Difference Between Electronegativity And Electron Affinity Definition Units Of Measurement Relationship With Atom Electron Affinity Ionic Bonding Chemistry
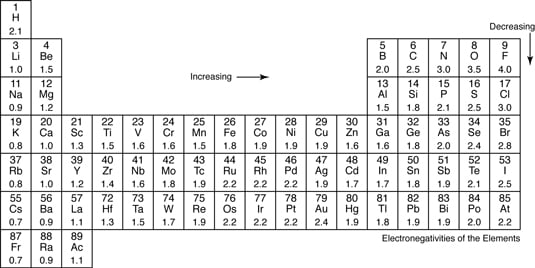
Electronegativity And Polar Covalent Bonding Dummies
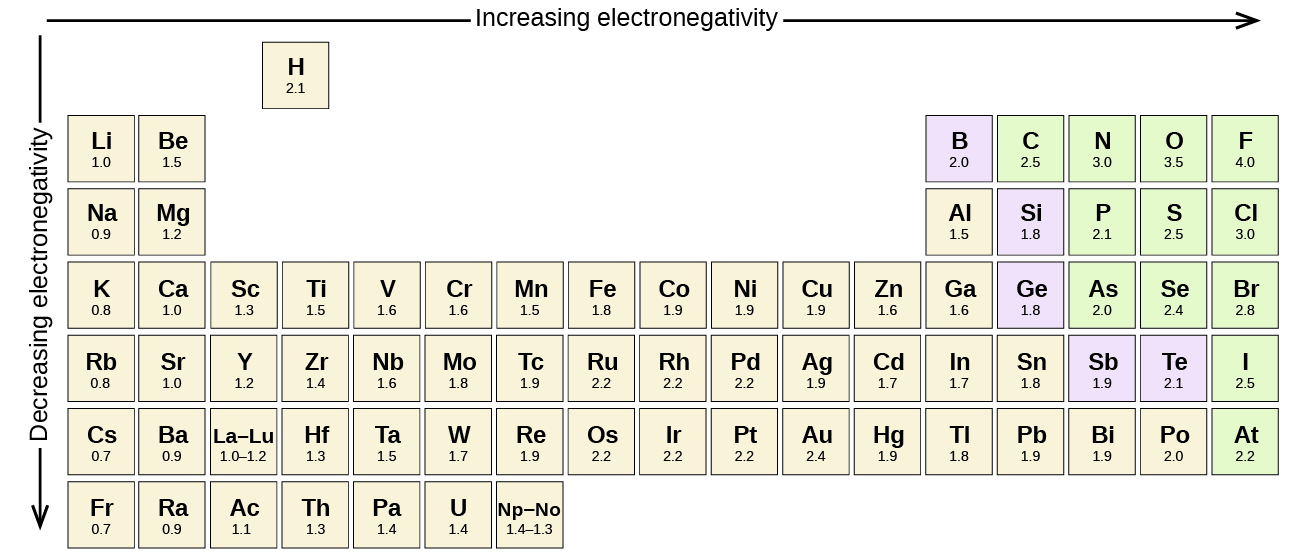
3 5 0 Electronegativity And Polarity Chemistry Libretexts

Electronegativity Measure Of An Atom S Ability To Attract Electrons To Itself In A Covalent Bond Nobl Chemistry Classroom Science Chemistry Chemistry Teacher
12 2 Electronegativity Chemistrysaanguyen

Electronegativity Table Chart Of The Elements Values Teaching Chemistry Chemistry Help Chemistry Lessons

Periodic Trends In Electronegativity Ck 12 Foundation Chemistry Periodic Table Ionization Energy Teaching Chemistry

3 Ways To Calculate Electronegativity Wikihow

Lesson Explainer Electronegativity Nagwa

Electronegativity Examples Trends Video Lesson Transcript Study Com

Trends In The Periodic Table Chpt 7 1 Atomic Radius Size 2 Ionization Energy 3 Electronegativity The Ionization Energy Periodic Table Covalent Bonding
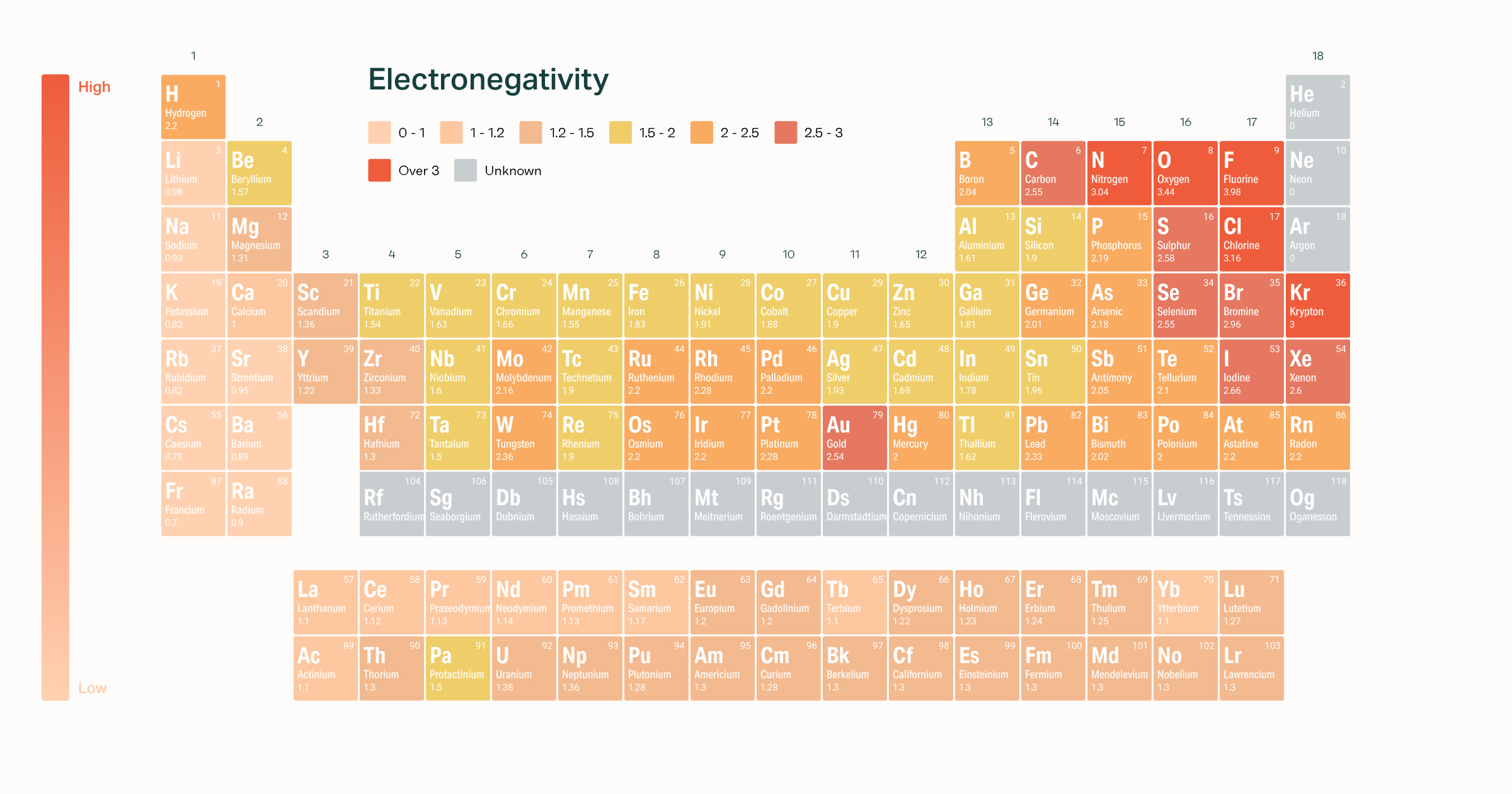
Electronegativity Of The Elements

Lesson Explainer Electronegativity Nagwa

Electronegativity Chart Periodic Table Chemistry Lessons Chemistry Basics
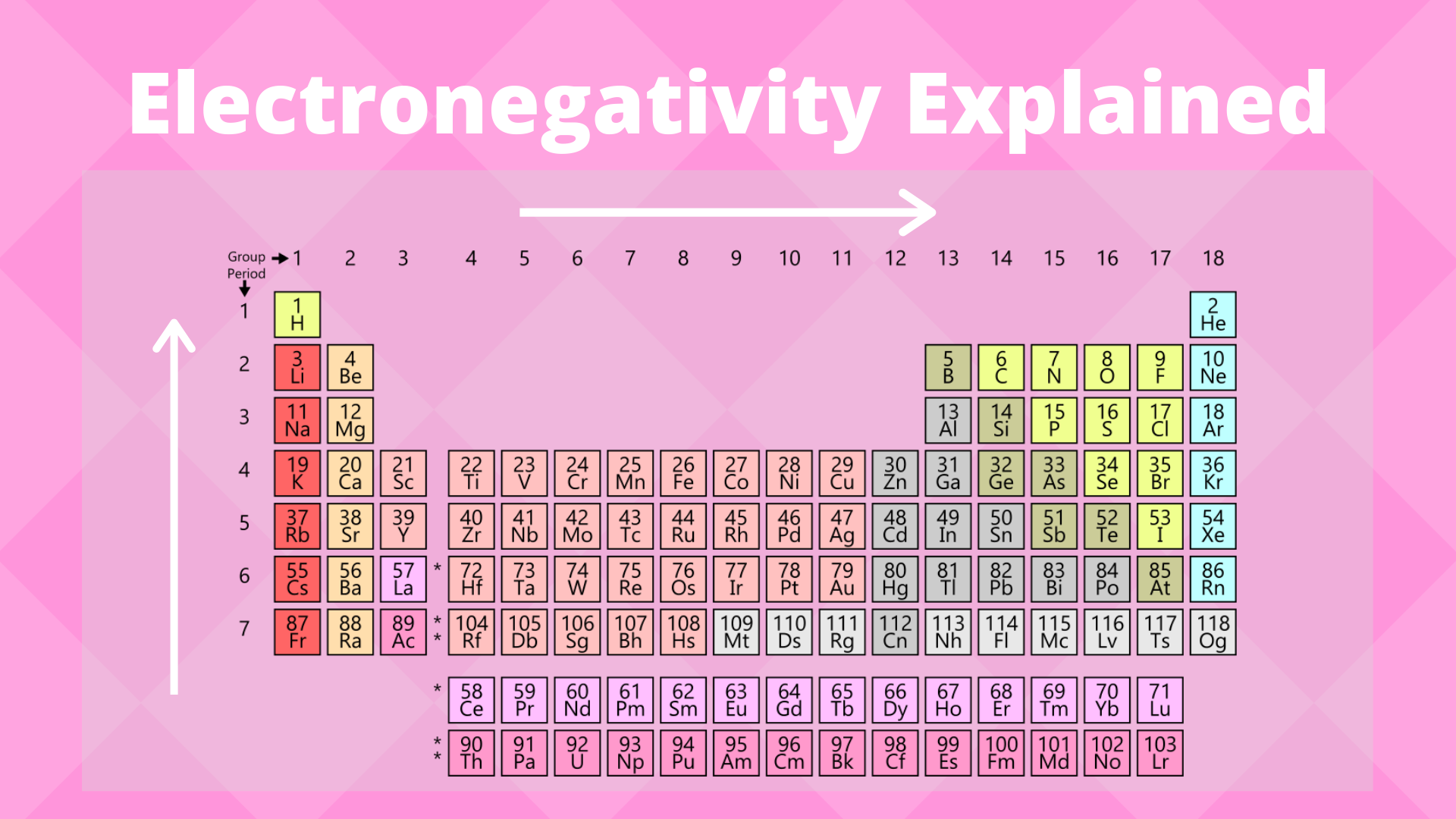
Electronegativity Trend Science Trends

Lesson Explainer Electronegativity Nagwa

Electronegativity Table Chart Of The Elements Values Template Propiedades Periodicas Apuntes De Clase Tabla Periodica

Electronegativity A Measure Of The Ability Of An Atom That Is Bonded To Another Atom To Attract Electrons To Itself Trend Increas Chemistry Atom Presentation

Comments
Post a Comment